Sds Surface Tension
Surface tension is relevant at larger scales as well, particularly in wetting, adhesion, and meniscus shape When the dimensions are in the micro to millimetre range, dynamics are given by a balance of viscosity, density, and surface tension Additionally, surface tension is an easily measured parameter for quality control.

Sds surface tension. The surface tensions of the 574S Chibowski et al/Adsorption Science & Technology Vol No 6 02 PVA– SDS and the PEG– SDS systems were measured using a thermostatted stalagmometer employing the ‘ free drop’ method (Lando et al 1967;. Surface Tension Test Ink (Red) 5971 Dynes/cm SDS Reference 05 Page 2 of 5 4 FIRST AID MEASURES 41 Description of measures Inhalation No special first aid measures necessary Skin contact Clean areas of skin affected with soap and plenty of water Eye contact Wash out eye thoroughly with plenty of water until irritation subsides. The surface tension of water changes as SDS is added, as shown in the graph below (a) Why does the surface tension of water change as SDS is added?.
This table highlights the viscosity and surface tension of a variety of solvents and other liquids An analogous table, which also includes Hansen Solubility Parameters and surface tension components, can be seen here If you are concerned with formulation problems which involve surface wetting, solubility, and viscosity control, you may wish. 50 °C, at an SDS concentration of 14 104 molâL1 and pH ) 4, the surface tension varies from 646 to 557 mNâm1AtpH) 12, surface tension values fall from 6 to 559 mNâm1 The surface tension of binary mixtures were correlated with temperature by the following expression (Jasper, 1972), proposed for pure components This equation has. GHS Safety Data Sheet Little Pro on Views Update Safety Data Sheet (SDS) is a very important document to inform its audience of the hazards of a chemical substance or mixture and provide advice on safety precautions Some countries may still call this document Material Safety Data Sheet (MSDS)In countries that have adopted GHS, Safety Data Sheet (SDS) will be the only.
For testing the surface tension To determine the cleanliness of surfaces Test Inks can make statements on the adhesive strength of printing inks, coatings and adhesives on all surfaces such as plastic, metal and glass as well as on the degree of cleanliness in mN/m (dyne/cm). For testing the surface tension To determine the cleanliness of surfaces Test Inks can make statements on the adhesive strength of printing inks, coatings and adhesives on all surfaces such as plastic, metal and glass as well as on the degree of cleanliness in mN/m (dyne/cm). ACCU DYNE TEST™ Surface Tension Test Fluids ACCU DYNE TEST™ surface tension test fluids are formulated per ASTM Standard D2578 from 100% reagent grade materials, and each batch is tensiometer tested to assure consistent results Learn more at the links below Test Procedure Product Info/Purchase MSDS.
Pierson and Witaker 1976). 22 22 Remarks for measuring surface tension Surface tension value is very sensitive to contamination. Samples of sodium dodecyl sulfarte (SDS) are commonly contaminated by dodecanol, as a minor component, which is very suface active and nt easily removed The desappearance of the minimum in the suface tension concentration curve at the dritical micelle condentration (CMC) gives no definite criterion about the purity of the sample.
The surface tension was reduced effectively when SDS blends with fluorocarbon surfactant, which reduces usage amount of fluorocarbon surfactant and cut the expenditure In best ratio of 06 07 conditions, the surface tension was the same with pure fluorocarbon surfactant. Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is a synthetic organic compound with the formula C H 3 (CH 2) 11 SO 4 NaIt is an anionic surfactant used in many cleaning and hygiene products This molecule is an organosulfate and a salt It consists of a 12carbon tail attached to a sulfate group, that is, it is the sodium salt of dodecyl. A study has been carried out on the modification of surface tension in aqueous solutions of the collector sodium dodecyl sulfate (SDS), at temperature of 293 K and pH between 4 and 11 The results of this study show that the surface tension varies very slightly when pH in the medium of flotation bath is modified.
A sensitive way to check the presence of dodecanol is to measure the surface tension of the solution, which will be lowered by the presence of the alcohol, especially just below the cmc The. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension, electrical conductivity, light scattering, and various spectroscopic technique 5 7 have been used to measure critical micelle concentration (CMC), aggregate size, and aggregation number of surfactants.
Colloidal unimolecular polymer particles, or CUPs, are true nanoscale charged particles of size less than 10 nm that are made by a simple method that allows for preparation of additivefree, zerovolatile organic content, and stable dispersions. Low surface tension makes it a particularly efficient rinse aid and low foam wetting agent in auto dish detergents, industrial and institutional cleaning, and in cleaningplace (CIP) applications such as food, dairy, and brewery cleaning It can be used in oilfield drilling and production formulations. The number of micelles asked by Anonymous on May 8, 13;.
Surface tension studiesSurface tension of SDS in water and in different percentage of maltodextrin (w/v) at 25 and 35 • C has been carried out by using Man Singh Survismeter The surface tension ( s ) is calculated by using the relation 19,s = 0 n w d s n s d w(6)where s is the surface tension of the solution, o the surface tension of the. The low density and low surface tension of DIBK enables formulators to develop highsolids coatings with low VOC content and excellent flow and leveling properties The chemical substances for this product are listed as Inert Ingredients Permitted for Use in Nonfood Use Pesticide Products under the Federal Insecticide, Fungicide, and. This table highlights the viscosity and surface tension of a variety of solvents and other liquids An analogous table, which also includes Hansen Solubility Parameters and surface tension components, can be seen here If you are concerned with formulation problems which involve surface wetting, solubility, and viscosity control, you may wish.
Surface tension is a property of liquids It is defined as the energy, or work, required to increase a liquid’s sur face area due to intermolecular forces. Surface additive for solventborne, solventfree and aqueous systems with a strong reduction in the surface tension Good wetting of critical substrates No influence on surface slip. Science You wish to make 1L of sterile lysis buffer soln that is 10mM TrisEDTA (pH 8) and 2% SDS.
The surface tension of water changes as SDS is added, as shown in the graph below (a) Why does the surface tension of water change as SDS is added?. Lower foam and dynamic surface tension values compared to the more hydrophophilic DYNOL™ 607 surfactant DYNOL™ 607 Surfactant Excellent choice as a replacement to conventional fluorosurfactants, good formulation compatibility DYNOL™ 800 Surfactant. The surface tension is determined by applying a stroke of ink measuring just a few centimetres in length to the surfaces to be evaluated and observing the behaviour of the stroke of ink If the stroke contracts within 2 or 4 seconds – depending on the ink specification – the surface tension of the test area is lower than that of the test ink.
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces Since these intermolecular forces vary depending on the nature of the liquid (eg water vs gasoline) or solutes in the liquid (eg surfactants like detergent), each solution exhibits differing surface tension properties. Test Pens filled with blue Test Ink are available from 28 to 72mN/m 30 to 72 mN/m according to ISO96, DIN Toxic from 24 to 57 mN/m;. Reduction of surface and interfacial tension When surfactants are dissolved in water they orientate at the surface so that the hydrophobic regions are removed from the aqueous environment, as shown in Figure 41a.
Surface tension measurements were also conducted for PEG400/SDS aqueous solutions, and results are shown in Fig 3 No break points were observed in the surface tension plots, and they show similar trends to polymerfree solution There is no interaction observed between PEG400 and SDS in the bulk solution. Surface tension is a property of liquids It is defined as the energy, or work, required to increase a liquid’s sur face area due to intermolecular forces. 31 Surface tension experiments When the surface tension of solutions with increasing concentration of surfactant is measured, the surface tension vs surfactant concentration plots show two straight lines, one with a steep negative slope and the other with a slightly decreasing one (Fig 1) The line with a steep linear decrease.
The dynamic surface adsorption properties of aqueous sodium dodecyl sulfate (SDS) solutions were investigated at different concentrations of NaCl using bubble pressure tensiometry MPTC In the case of ionic surfactants, the existence of a diffuse electric double layer on the surface adsorption layer and around the micelle produces a surface charge. For surface tension components (eg dispersive and polar, hydrogen bonding, acidbase contributions etc), corresponding (single) references and data at elevated temperatures up to 180 °C, and surfactant property prediction (CMC, surface tension@CMC), please send an email to the address below. Surface tension isotherms, adsorption dynamics and dilational viscoelasticity of sodium dodecyl sulphate solutions Colloids and Surfaces A Physicochemical and Engineering Aspects 10 , 354 (13) , 815.
If surface tension experiments are performed (CMC 2), use the Gibbs equation to calculate the surface excess concentration, Γ of SDS at the cmc Hence calculate the average area occupied by each adsorbed dodecyl sulphate ion at the airwater interface From your knowledge of bond. After 24 s, the wettability of the surface reached a value and remains constant This behavior suggests that cement adhesion to the rock surface will be quick and possibly efficient Figure 4 shows the contact angle behavior with time variation of saturated limestone with SDSformulated flushing fluids. From dynamic surface tension data the micelle kinetics dissolution constants for SDS–DTAB mixed solutions of the two components and the highly surface active complex are determined The experiments at different mixing ratios 1∶10, 1∶1 and 10∶1 allow conclusions about the competitive adsorption of the complex.
The number of micelles asked by Anonymous on May 8, 13;. Available in a range of 184 to 105 mN/m surface tension;. The surface tension of Intechem01 SDS composite surfactants and its changes with temperature, concentration and proportion has been investigated The aim of the study has been to reveal the essence of surfactant effects, which is significance to find the relationship between gas hydrate formation rate and surfactant kinds, dosage and.
The number of micelles asked by Anonymous on May 8, 13;. From dynamic surface tension data the micelle kinetics dissolution constants for SDS–DTAB mixed solutions of the two components and the highly surface active complex are determined. This table is provided as an information service by Diversified Enterprises This table shows the dispersion (nonpolar) and polar components, as well as an acid—base breakout, of surface tension for a variety of solvents and other liquids which are commonly used in the analysis of solid state surface energy.
SDS is a detergent, an anionic (negatively charged) surfactant (compound that lowers surface tension) In the case of proteins, SDS disrupts the noncovalent bonds in protein molecules PAGE is a biochemical technique that allows for proteins to be separated by their electrophorectic mobility (how fast they move in an electric field) In the. The surface tension was reduced effectively when SDS blends with fluorocarbon surfactant, which reduces usage amount of fluorocarbon surfactant and cut the expenditure In best ratio of 06 07 conditions, the surface tension was the same with pure fluorocarbon surfactant. Fluorosurfactants can reduce the surface tension of water down to a value half of what is attainable by using hydrocarbon surfactants This ability is due to the lipophobic nature of fluorocarbons, as fluorosurfactants tend to concentrate at the liquidair interface.
The surface tension and interfacial tension therefore depend on how much time has elapsed since a new phase boundary was formed Accordingly, the interfacial tension or surface tension can be considerably higher with dynamic processes than in the steady state This situation significantly affects the right choice and dosage of a surfactant. The equilibrium surface tension of solutions containing SDS in water was measured with the increasing concentration of surfactant, then the same procedure was repeated for the HCl – SDS systems to find HCl electrolyte and heavy metal ions effects on the surface tension and CMC values of the systems containing SDS. The surface tension of water changes as SDS is added, as shown in the graph below (a) Why does the surface tension of water change as SDS is added?.
Thus, the surface tension formula is Surface tension = (surface force)/ (length force acts) γ = F /d Over here Γ refers to the Surface tension F is the force which applies to the liquid d refers to the length where the force acts Solved Examples on Surface Tension Question You have a small piece of metal that is 1 cm long and weighs 0. A bubble pressure tensiometer was also used to characterize the surface tension of the aqueous polyol (00 wt %) solution, the sodium dodecyl sulfate (SDS, 050 wt %) solution, and the ink This. ACCU DYNE TEST™ Surface Tension Test Fluids ACCU DYNE TEST™ surface tension test fluids are formulated per ASTM Standard D2578 from 100% reagent grade materials, and each batch is tensiometer tested to assure consistent results Learn more at the links below Test Procedure Product Info/Purchase MSDS.
Surface tension is defined as the molecular force at the interface of two immiscible mediums The intermolecular attractive forces between the molecules at the interface (also known as surface energy) causes a tension between two fluids 11, 12. Surface tension for aqueous solutions of sodium dodecyl sulfate (SDS) are shown in Table 1, according to pH at temperatures ranging between °C and 50 °C and at surfactant concentrations of between 73 106molâL1 and 14 104molâL1 The values show that surface * To whom correspondence should be addressed. Reduction of surface and interfacial tension When surfactants are dissolved in water they orientate at the surface so that the hydrophobic regions are removed from the aqueous environment, as shown in Figure 41a.
Surface Tension, Viscosity, and Refractive Index of Sodium Dodecyl Sulfate (SDS) in Aqueous Solution Containing Poly (ethylene glycol) (PEG), Poly (vinyl pyrrolidone) (PVP), and Their Blends Journal of Chemical & Engineering Data 17, 62 (7), https//doiorg//acsjced6b Imre Varga and Richard A Campbell. Science You wish to make 1L of sterile lysis buffer soln that is 10mM TrisEDTA (pH 8) and 2% SDS. From dynamic surface tension data the micelle kinetics dissolution constants for SDS–DTAB mixed solutions of the two components and the highly surface active complex are determined The experiments at different mixing ratios 1∶10, 1∶1 and 10∶1 allow conclusions about the competitive adsorption of the complex.
Science You wish to make 1L of sterile lysis buffer soln that is 10mM TrisEDTA (pH 8) and 2% SDS. The timedependent variation in the surface tension can be elucidated by considering the transport dynamics As schematically illustrated in Figure 2c, initially, the release of the SDS molecules creates a surface tension valley sufficient to initiate Marangoni flow However, the continuous adsorption of SDS at the air/water interfaces also. Surface tension is the attractive force found in liquids which is responsible for pulling surface molecules in the rest of the liquid Further, it minimizes the surface area The attractive forces we are talking about here are because of electrostatic forces.
The classic case is for SDS, which is easily hydrolyzed to dodecanol Adsorption of this cosurfactant produces a minimum in surface tension just above the cmc The lack of a minimum is good. The surface tension was reduced effectively when SDS blends with fluorocarbon surfactant, which reduces usage amount of fluorocarbon surfactant and cut the expenditure In best ratio of 06 07 conditions, the surface tension was the same with pure fluorocarbon surfactant. The surface tension, surface excess and surface concentration (defined as the amount of surfactant adsorbed in the surface phase divided by the surface area) of two anionic surfactants, namely dodecyl sulfate sodium and dodecyl sulfate caesium, dissolved in nonaqueous polar solvent formamide have been separately measured at 6 °C through independent experiments.
The surface tension is an intensive thermodynamic quantity, which is very important for the determination of cmc of surfactant On addition of a surfactant, surface tension of solution decreases and at a particular concentration, the surface tension of the solution tends to remain constant. Low surface tension makes it a particularly efficient rinse aid and low foam wetting agent in auto dish detergents, industrial and institutional cleaning, and in cleaningplace (CIP) applications such as food, dairy, and brewery cleaning It can be used in oilfield drilling and production formulations. Surface tension, electrical conductivity, light scattering, and various spectroscopic technique 5 7 have been used to measure critical micelle concentration (CMC), aggregate size, and aggregation number of surfactants.
21 Data examples of dynamic surface tension Dynamic surface tension of 4 concentration level of SDS solution Ability of lowering surface tension does not progress in the range of concentration more than 05wt% Dynamic CMC?. The existence of a minimum in the surface tension (γ)−concentration (C) curve of surfactant solutions is investigated Equilibrium surface tension and solution conductivity (κ) of anionic surfactant (SDS) solution and mixed anionic−nonionic surfactant (SDS−NP (EO) 40) solutions are measured Minima are observed in the γ−log C curves.
Pubs Acs Org Doi Pdf 10 1021 Acs Jced 6b

Table 1 From Surfactant Surface Tension Effects On Promoting Hydrate Formation An Experimental Study Using Fluorocarbon Surfactant Intechem 01 Sds Composite Surfactant Semantic Scholar

Effect Of Surfactants On The Rate Of Diffusion Controlled Anodic Dissolution Of Copper In Orthophosphoric Acid
Sds Surface Tension のギャラリー

Self Assembly Of Sodium Dodecylsulfate And Dodecyltrimethylammonium Bromide Mixed Surfactants With Dyes In Aqueous Mixtures Royal Society Open Science

Self Assembly Of Sodium Dodecylsulfate And Dodecyltrimethylammonium Bromide Mixed Surfactants With Dyes In Aqueous Mixtures Royal Society Open Science
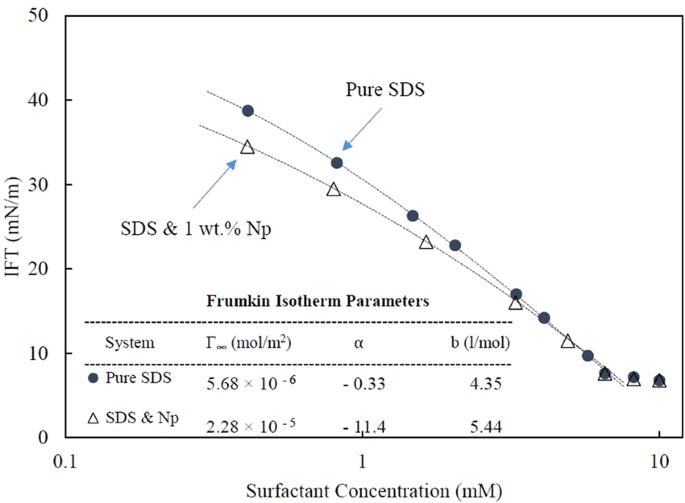
The Role Of Electrostatic Repulsion On Increasing Surface Activity Of Anionic Surfactants In The Presence Of Hydrophilic Silica Nanoparticles Scientific Reports

Effect Of Methanol On The Surface Tension And Viscosity Of Sodiumdodecyl Sulfate Sds In Aqueous Medium At 298 15 323 15 K Sciencedirect

Figure 3 From Adsorption Layer Characteristics Of Mixed Sodium Dodecyl Sulfate C N Eo M Solutions 1 Dynamic And Equilibrium Surface Tension Semantic Scholar
2

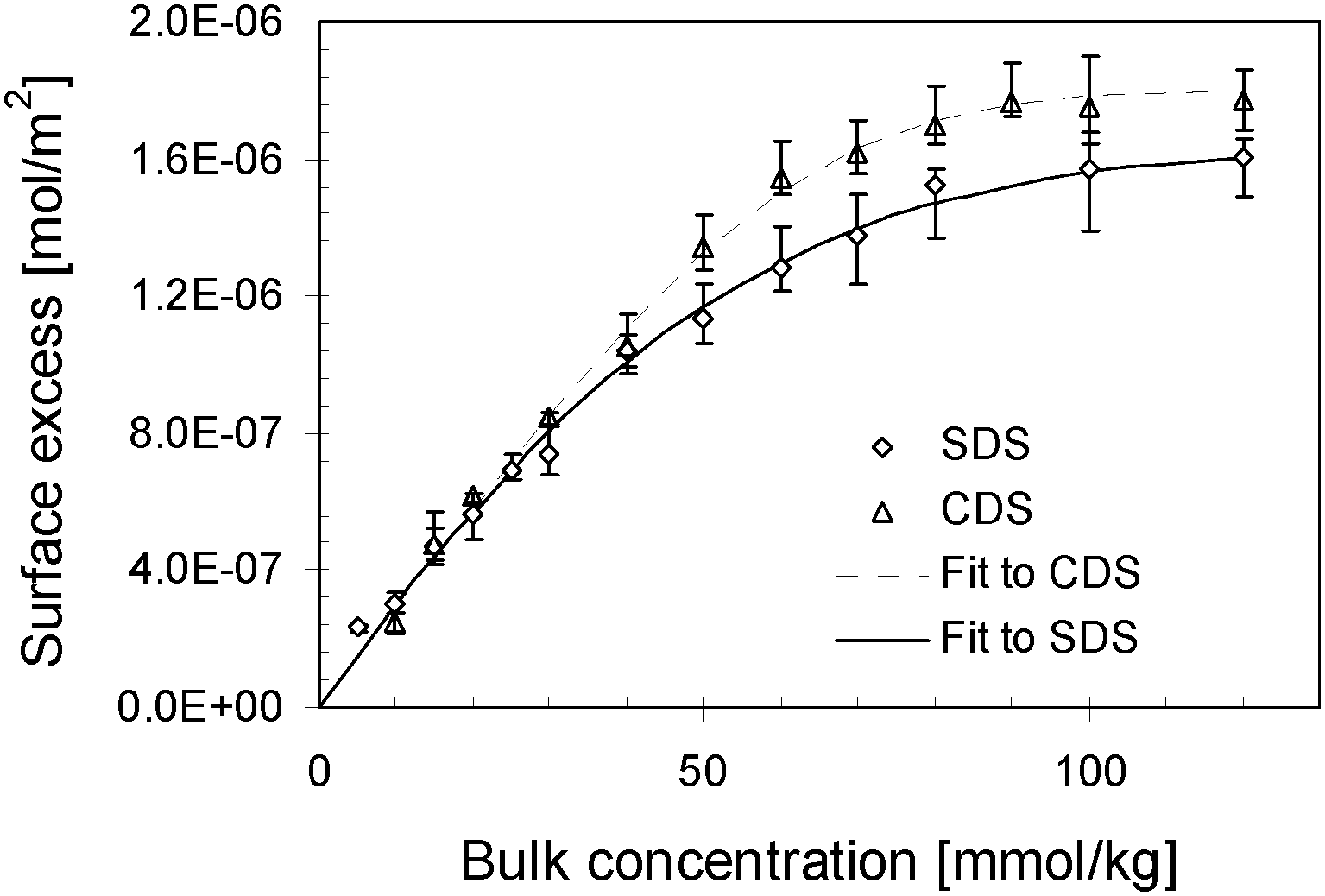
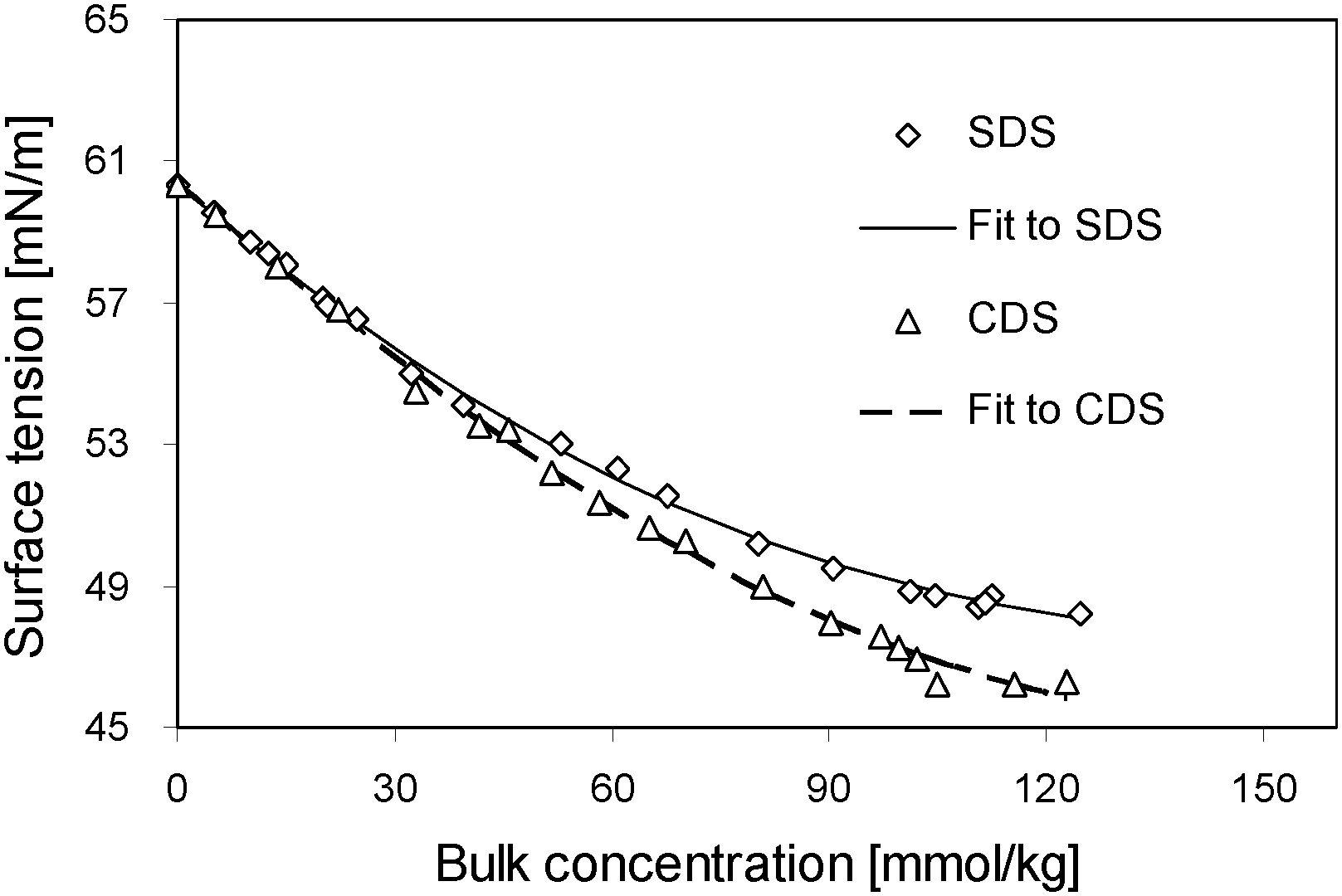
Surface Tension Of Sds And Cds As A Function Of Surface Excess The Download Scientific Diagram

Surface Tension Interfacial Tension And Emulsification Of Sodium Dodecyl Sulfate Extended Surfactant Sciencedirect
Http Www Jsirjournal Com Vol5 Issue6 03 Pdf
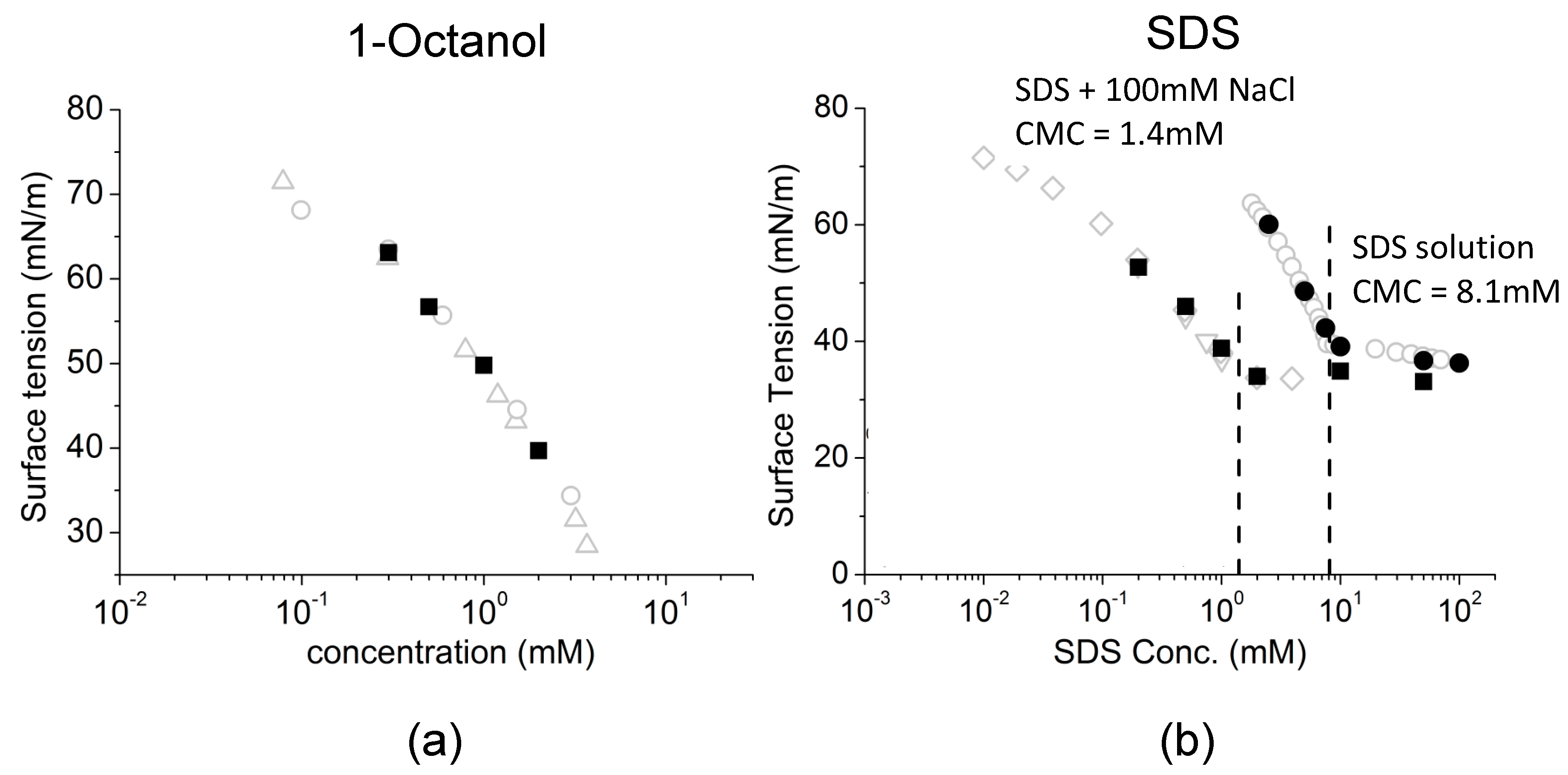
The Dependence Of Surface Tension On Surface Properties Of Ionic Surfactant Solution And The Effects Of Counter Ions Therein Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cpg

Influence Of Soluble Surfactant Properties On The Activation Of Aerosol Particles Containing Inorganic Solute In Journal Of The Atmospheric Sciences Volume 55 Issue 10 1998

Critical Surface Tension And Contact Angle With Water For Various Polymers Sort By Contact Angle
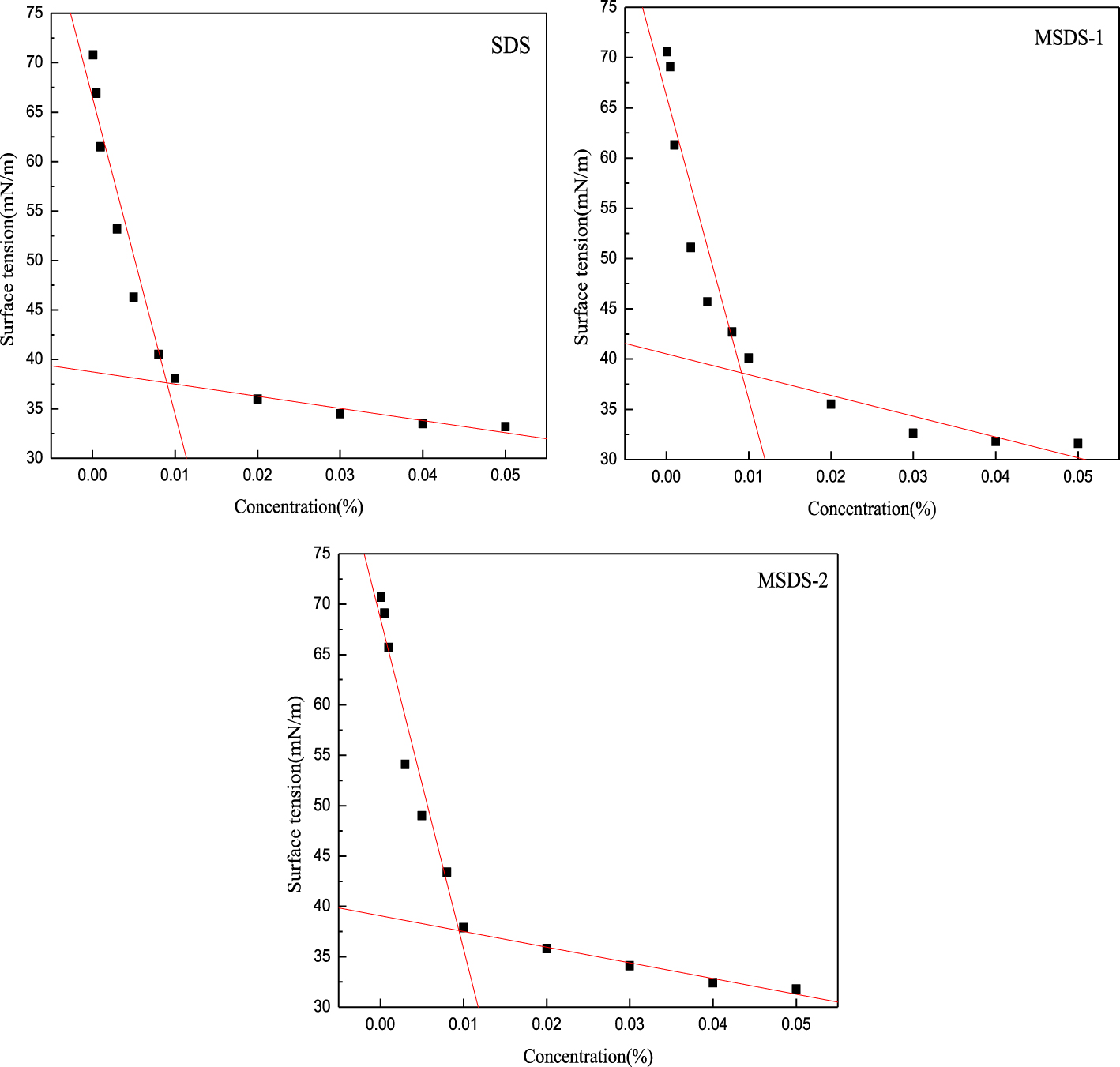
Comptes Rendus Chimie
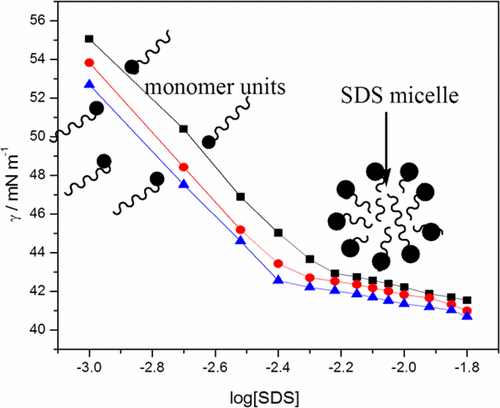
Surface Tension Viscosity And Refractive Index Of Sodium Dodecyl Sulfate Sds In Aqueous Solution Containing Poly Ethylene Glycol Peg Poly Vinyl Pyrrolidone Pvp And Their Blends Journal Of Chemical Engineering Data X Mol

The Dependence Of Surface Tension On Surface Properties Of Ionic Surfactant Solution And The Effects Of Counter Ions Therein Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cpg

Determination Of The Stabilization Time Of The Solution Air Interface For Aggregates Formed By Nac In Mixtures With Sds And Peo Investigated By Dynamic Surface Tension Measurements

Effect Of Tbab And Sds Surfactants On The Interfacial Tension Of Co2 Hydrate In Water
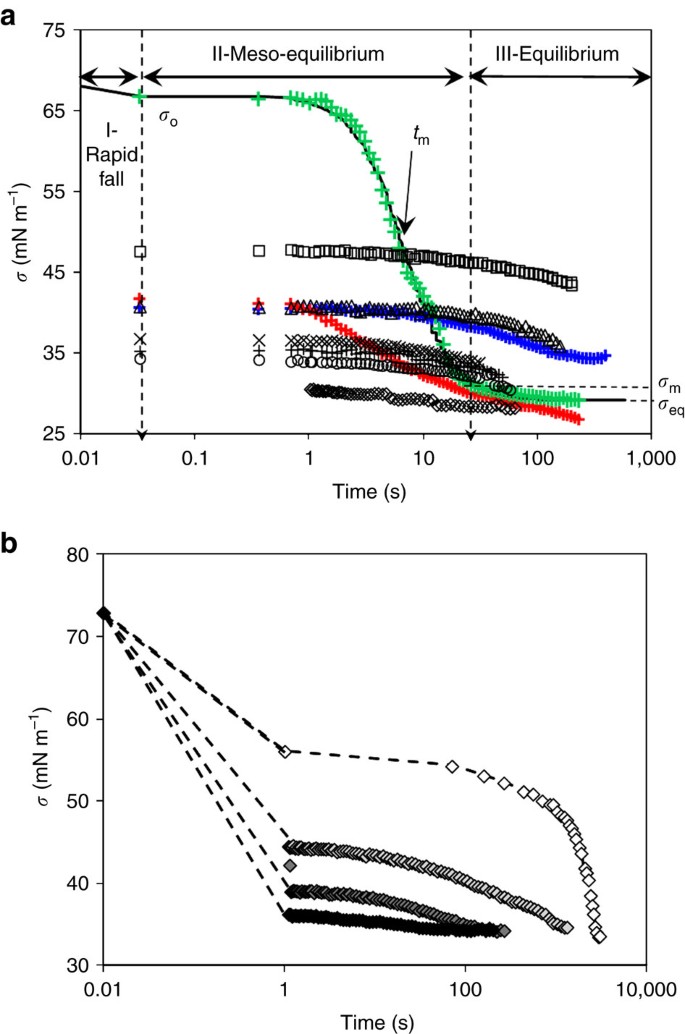
The Dynamic Surface Tension Of Atmospheric Aerosol Surfactants Reveals New Aspects Of Cloud Activation Nature Communications

Interaction Of Nonionic Surfactant Aeo9 With Ionic Surfactants Abstract Europe Pmc

Surface Tension And Adsorption Studies By Drop Profile Analysis Tensiometry Kairaliyeva 17 Journal Of Surfactants And Detergents Wiley Online Library
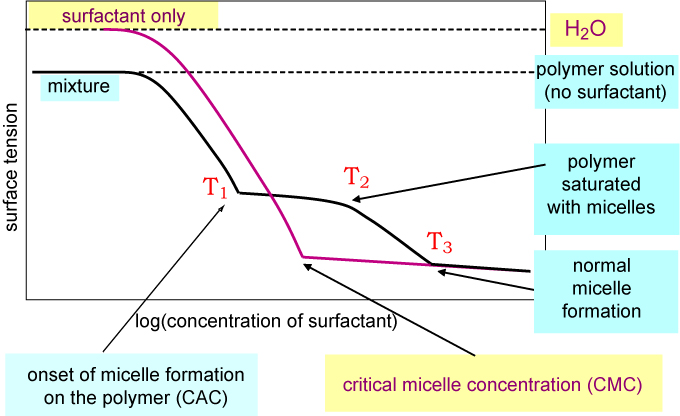
The Thomas Penfold Group Ptcl Oxford

Insights On Structuration Of Peroxotungstic Acid In Aqueous Media

Effect Of Ethanol On Partition And Binding Equilibrium Of Phenothiazine In Anionic And Nonionic Micellar Solutions Scialert Responsive Version
Arxiv Org Pdf 1610
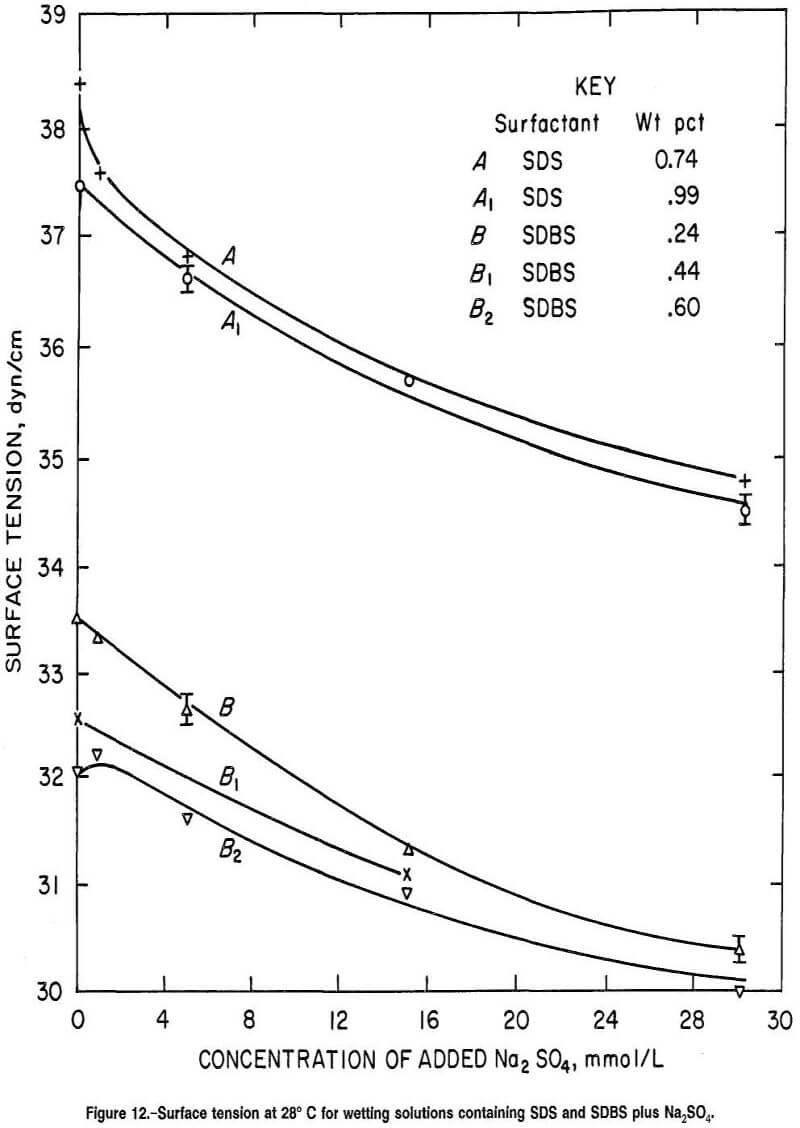
Sulfate Ion Anionic Surfactants
Http Eprints Lincoln Ac Uk 281 2 281 acs Langmuir 5b Pdf
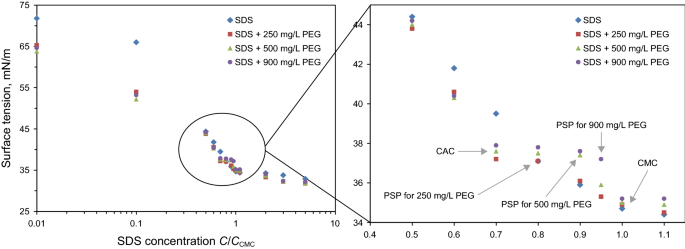
Novel Application Of Peg Sds Interaction As A Wettability Modifier Of Hydrophobic Carbonate Surfaces Springerlink
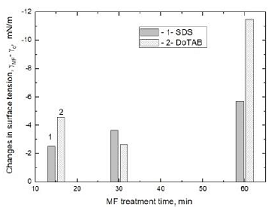
Magnetic Field Effects Surface Tension Edelweiss Chemical Science
Q Tbn And9gctwqjdqt9c9sfsy7pxslxfv1svveht3nrfqkzzemtdpxjciabao Usqp Cau
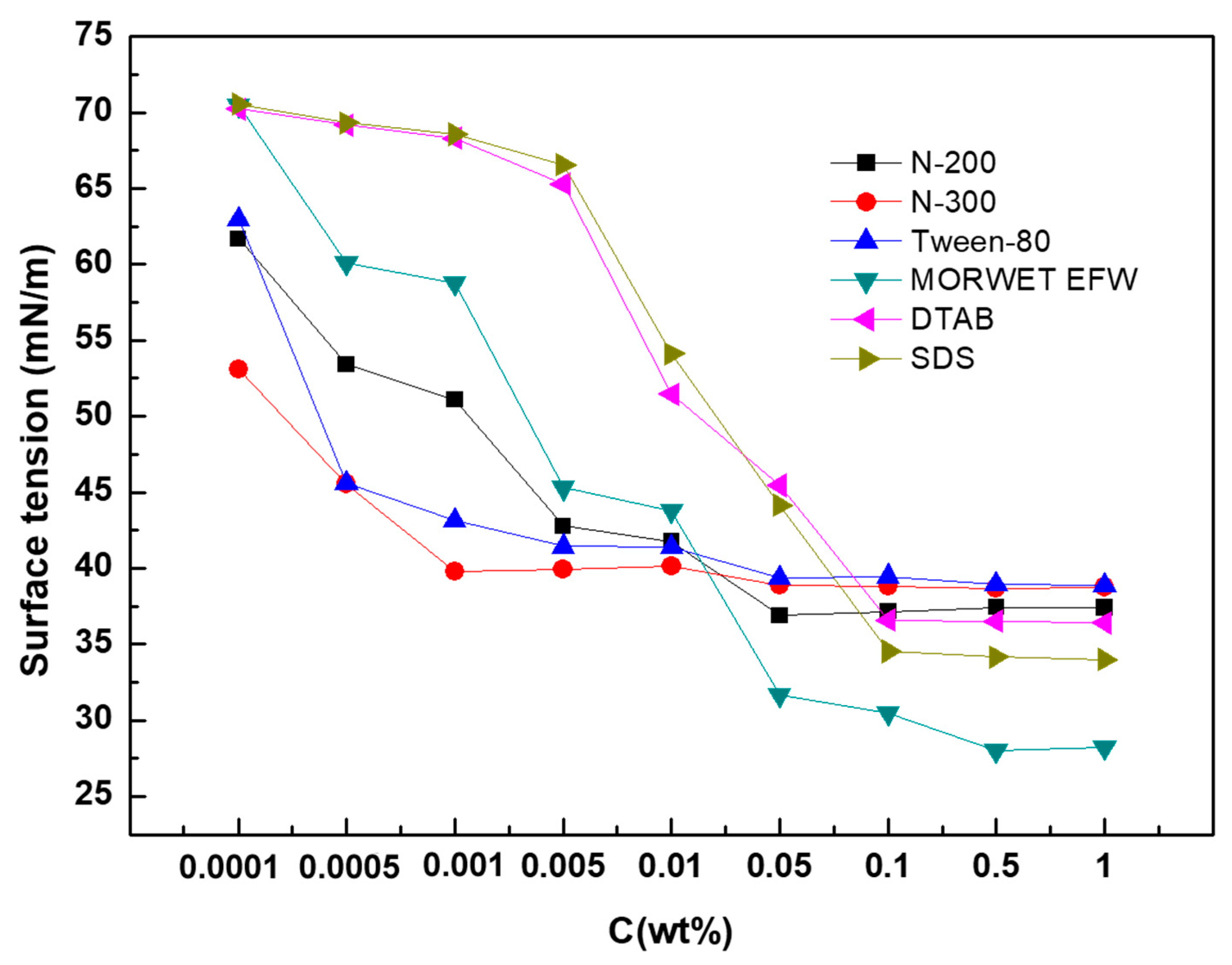
Molecules Free Full Text Wetting Behavior And Maximum Retention Of Aqueous Surfactant Solutions On Tea Leaves Html

Surface Tension What Is Surface Tension Kibron

Sodium Dodecyl Sulphate Sds Residue Analysis Of Foam Formed Cellulose Based Products In Nordic Pulp Paper Research Journal Volume 35 Issue 2
The Dependence Of Surface Tension On Surface Properties Of Ionic Surfactant Solution And The Effects Of Counter Ions Therein Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cpg

Effect Of Ethanol On Partition And Binding Equilibrium Of Phenothiazine In Anionic And Nonionic Micellar Solutions Scialert Responsive Version

Micellization Behaviour Of Sodium Dodecyl Sulphate In Presence And Absence Of Sodium Sulphate And Zinc Sulphate In Distilled Water By Surface Tension Measurement

Self Assembly Of Sodium Dodecylsulfate And Dodecyltrimethylammonium Bromide Mixed Surfactants With Dyes In Aqueous Mixtures Royal Society Open Science

Maltodextrin Sds Interactions Volumetric Viscometric And Surface Tension Study Sciencedirect

The Surface Tension Of Foam Versus Sds Concentration Download Scientific Diagram
Micromachines Free Full Text Micro Surface And Interfacial Tensions Measured Using The Micropipette Technique Applications In Ultrasound Microbubbles Oil Recovery Lung Surfactants Nanoprecipitation And Microfluidics Html

Surfactant Surface Tension Effects On Promoting Hydrate Formation An Experimental Study Using Fluorocarbon Surfactant Intechem 01 Sds Composite Surfactant
Kundoc Com Download Water Interface 5aed3813d64ab2f8b2f1b4f6 Html

Study Of Surface Properties Of Aqueous Solutions Of Sodium Dodecyl Sulfate In The Presence Of Hydrochloric Acid And Heavy Metal Ions Sciencedirect

Effect Of Glycerol With Sodium Chloride On The Krafft Point Of Sodium Dodecyl Sulfate Using Surface Tension Sciencedirect
3

Surface Activity And Foaming Capacity Of Aggregates Formed Between An Anionic Surfactant And Non Cellulosics Leached From Wood Fibers Abstract Europe Pmc
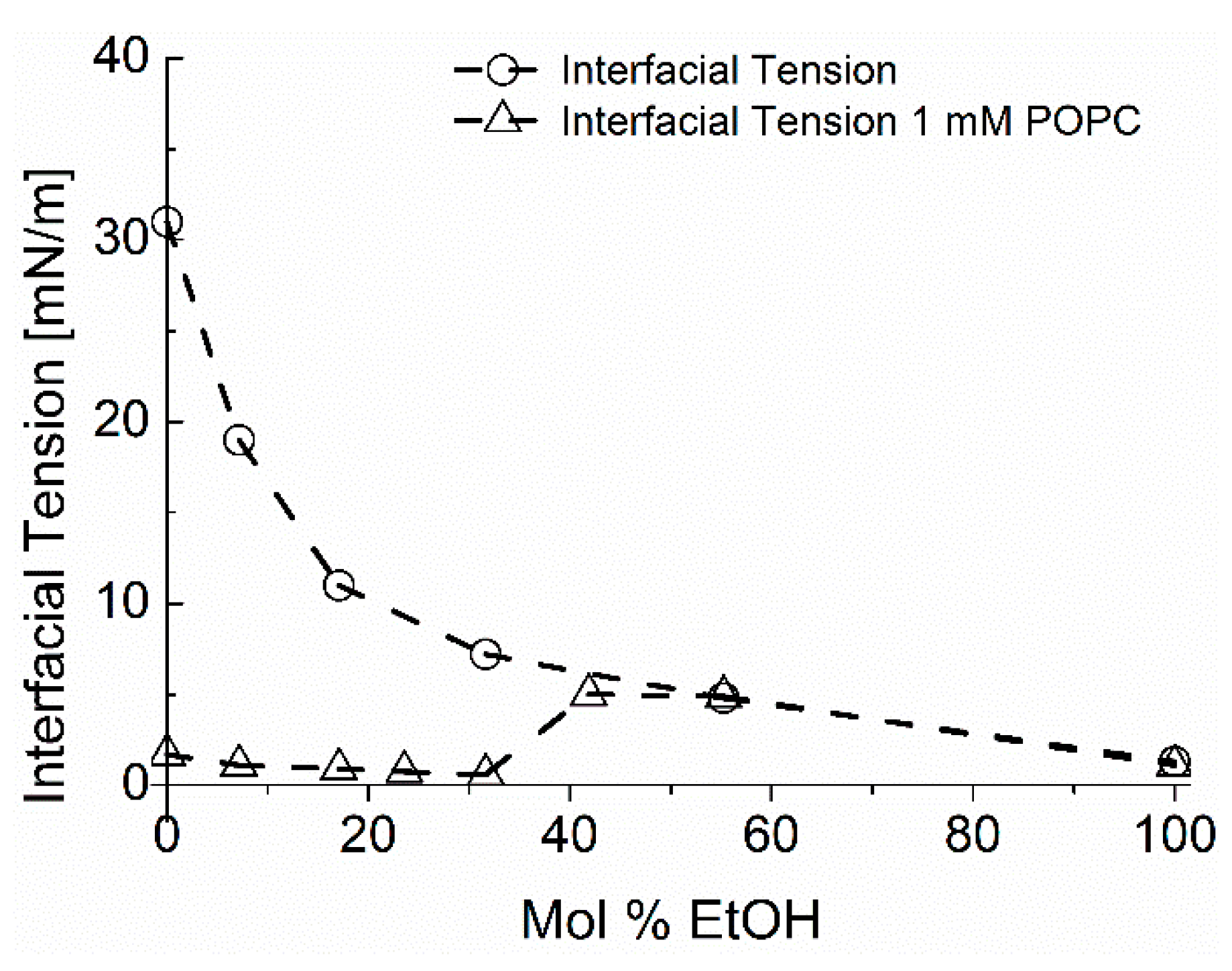
Micromachines Free Full Text Micro Surface And Interfacial Tensions Measured Using The Micropipette Technique Applications In Ultrasound Microbubbles Oil Recovery Lung Surfactants Nanoprecipitation And Microfluidics Html

Recovery Of Crude Oil By Chemical Flooding Method Using Sds And Gum Arabic Mixtures Oriental Journal Of Chemistry
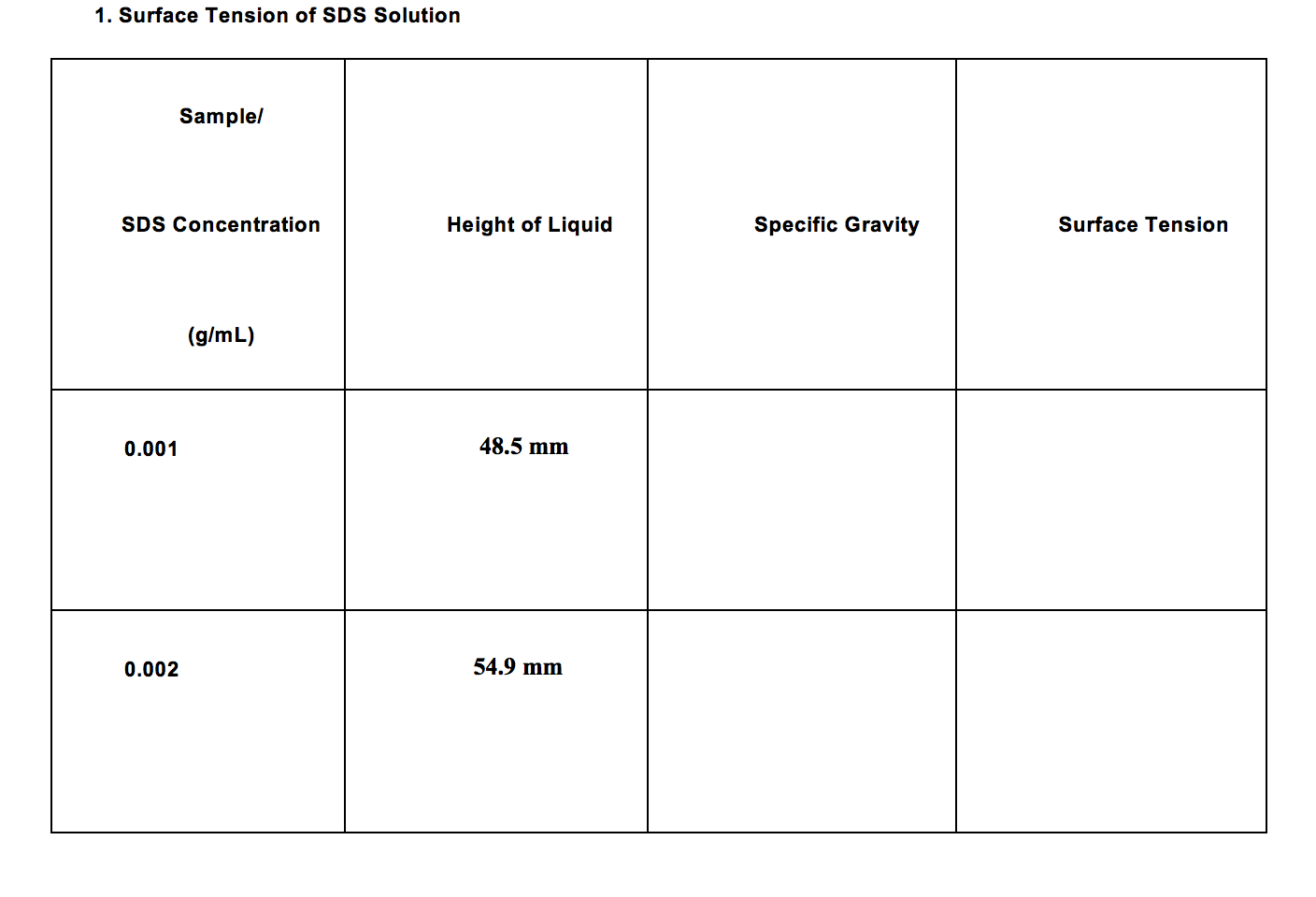
Solved 1 Surface Tension Of Sds Solution Sample Sds Conc Chegg Com
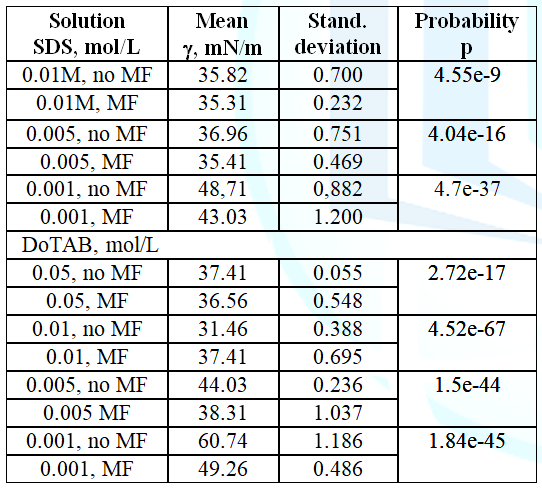
Magnetic Field Effects Surface Tension Edelweiss Chemical Science

Influence Of Soluble Surfactant Properties On The Activation Of Aerosol Particles Containing Inorganic Solute In Journal Of The Atmospheric Sciences Volume 55 Issue 10 1998

Analysis Of Dynamic Surface Tension Data For Sds Dtab Mixed Solutions Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 130a

Surfactant Surface Tension Effects On Promoting Hydrate Formation An Experimental Study Using Fluorocarbon Surfactant Intechem 01 Sds Composite Surfactant

Pdf Micellization Behaviour Of Sodium Dodecyl Sulphate In Presence And Absence Of Sodium Sulphate And Zinc Sulphate In Distilled Water By Surface Tension Measurement Ajaya Bhattarai Academia Edu
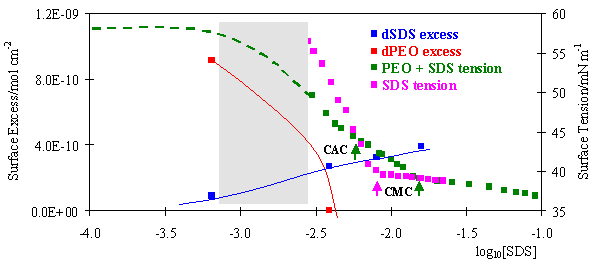
The Thomas Penfold Group Ptcl Oxford

A New Simple Method For Determining The Critical Micelle Concentration Of Surfactants Using Surface Plasmon Resonance Of Silver Nanoparticles Journal Of Analytical Science And Technology Full Text
1
Www Hanser Elibrary Com Doi Pdf 10 3139 113
2
Www Journal Csj Jp Doi Pdf 10 1246 sj 32 646
Http Tetrazolelover At Ua Unsorted New Doukkali17 Pdf

Self Assembly Of Sodium Dodecylsulfate And Dodecyltrimethylammonium Bromide Mixed Surfactants With Dyes In Aqueous Mixtures Royal Society Open Science

Discrepancies Over The Onset Of Surfactant Monomer Aggregation Interpreted By Fluorescence Conductivity And Surface Tension Methods

Equilibrium Surface Tension Versus Sds Aqueous Solution For Different Download Scientific Diagram
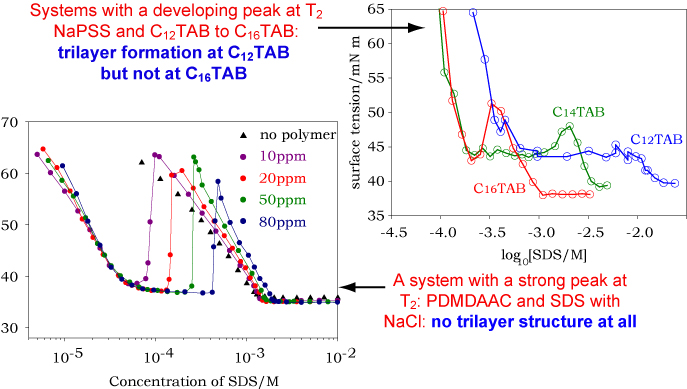
The Thomas Penfold Group Ptcl Oxford

Measurement Of Dynamic Surface Tension By Mechanically Vibrated Sessile Droplets Review Of Scientific Instruments Vol 87 No 4

Sodium Dodecyl Sulphate Sds Residue Analysis Of Foam Formed Cellulose Based Products In Nordic Pulp Paper Research Journal Volume 35 Issue 2
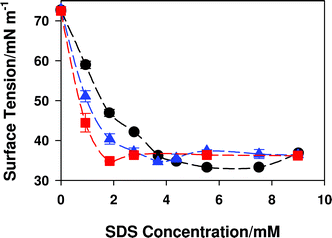
Influences Of Surfactant And Nanoparticle Assembly On Effective Interfacial Tensions Physical Chemistry Chemical Physics Rsc Publishing
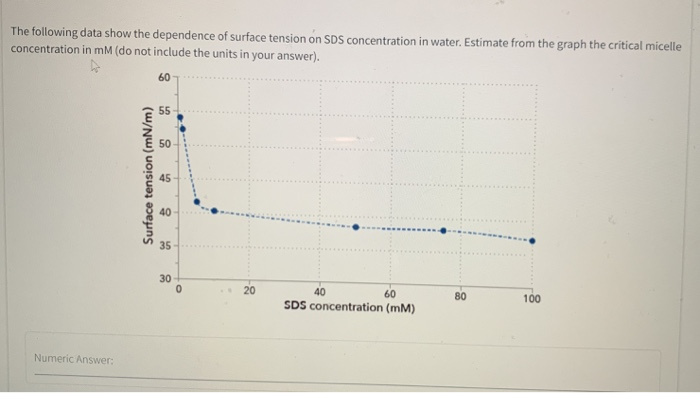
Solved The Following Data Show The Dependence Concentrati Chegg Com

Figure 1 From Surfactant Surface Tension Effects On Promoting Hydrate Formation An Experimental Study Using Fluorocarbon Surfactant Intechem 01 Sds Composite Surfactant Semantic Scholar
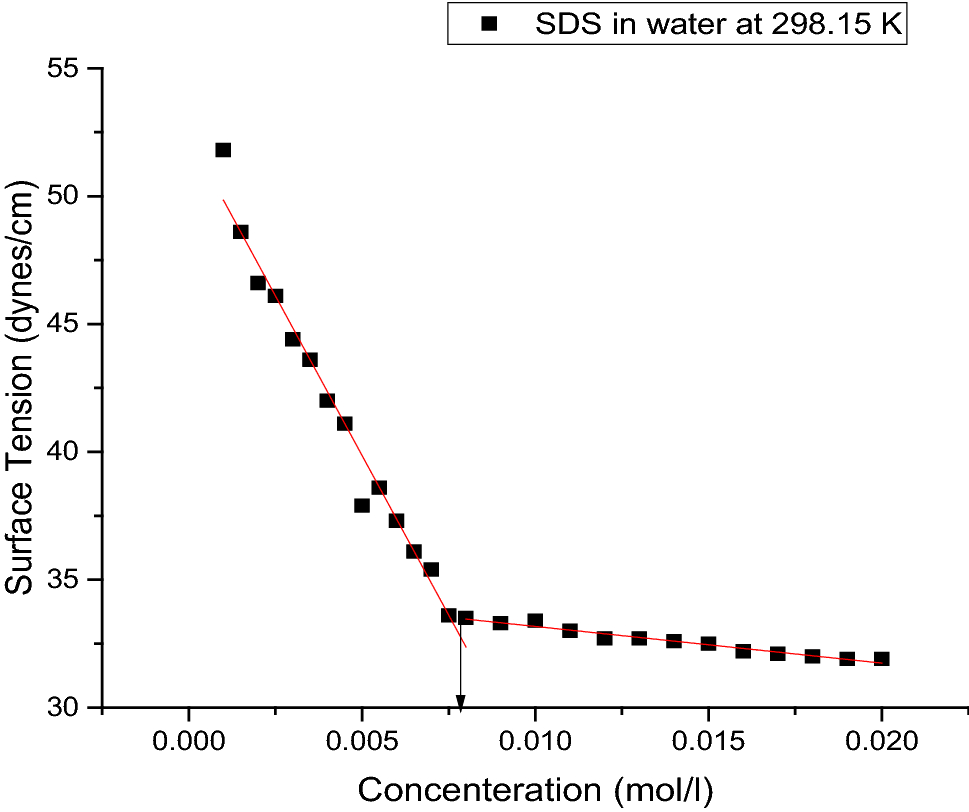
Figure 9 Solvation Thermodynamic Parameters For Sodium Dodecyl Sulfate Sds And Sodium Lauryl Ether Sulfate Sles Surfactants In Aqueous And Alcoholic Aqueous Solvents Springerlink

Association Of Branched Polyethylene Imine With Surfactants In Aqueous Solution
Www Ijrte Org Wp Content Uploads Papers V7i6s5 Fs519 Pdf

Surface Tension Isotherms Of Sds In The Presence Of Nacl At Different Download Scientific Diagram
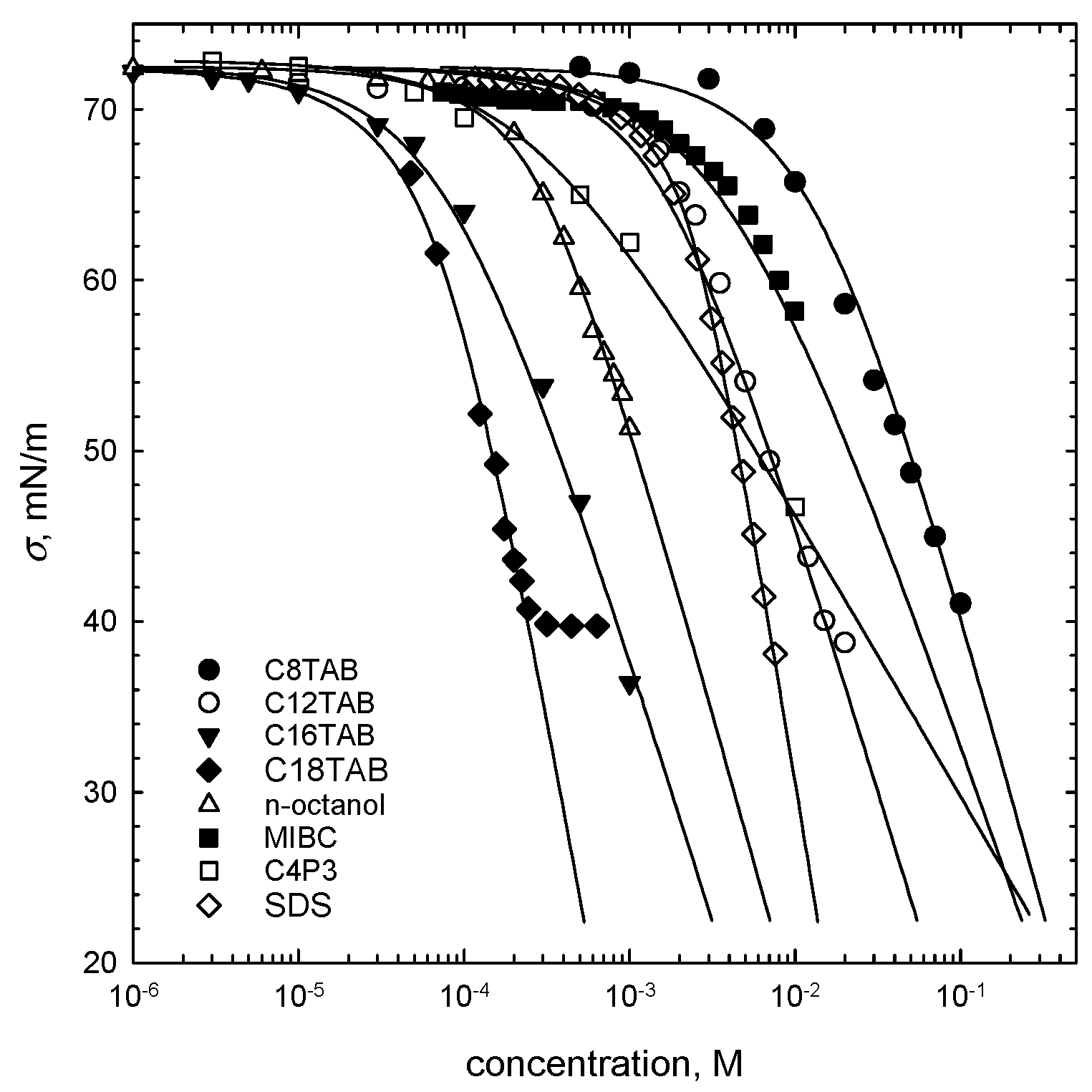
Minerals Free Full Text Critical Synergistic Concentration Of Binary Surfactant Mixtures Html
Q Tbn And9gcst43wyniobbpe1vxqeigxycohjkw2eyiyhlkhvmp64w0eikf3k Usqp Cau

Surface Tension G Vs Log Surfactant For Pure Sds And Sdbs At 303 K Download Scientific Diagram

Dependence Of Surface Tension On Concentration In Sds Aqueous Solutions Download Scientific Diagram
Http Dro Dur Ac Uk 1 Pdf

Surface Tension And Adsorption Studies By Drop Profile Analysis Tensiometry Kairaliyeva 17 Journal Of Surfactants And Detergents Wiley Online Library

Influence Of Soluble Surfactant Properties On The Activation Of Aerosol Particles Containing Inorganic Solute In Journal Of The Atmospheric Sciences Volume 55 Issue 10 1998
2
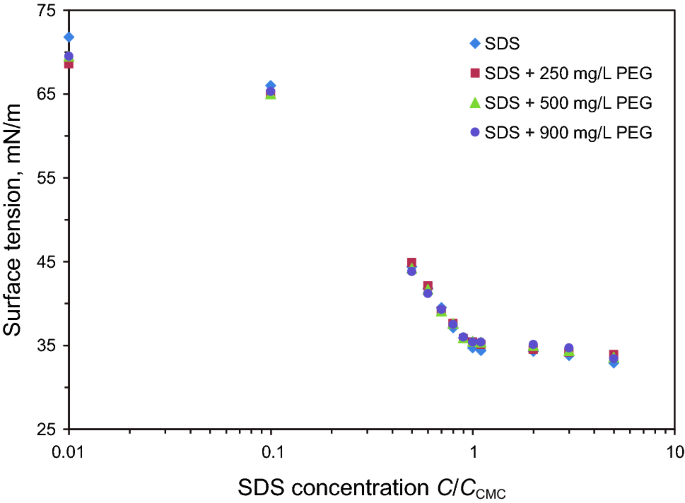
Novel Application Of Peg Sds Interaction As A Wettability Modifier Of Hydrophobic Carbonate Surfaces Springerlink

Discrepancies Over The Onset Of Surfactant Monomer Aggregation Interpreted By Fluorescence Conductivity And Surface Tension Methods
Cecas Clemson Edu Kornevlab Article 3 Pdf

An Investigation Of Pulmonary Surfactant Physicochemical Behavior Under Airway Reopening Conditions Journal Of Applied Physiology

An Investigation Of Pulmonary Surfactant Physicochemical Behavior Under Airway Reopening Conditions Journal Of Applied Physiology

Effect Of Methanol On The Surface Preview Related Info Mendeley
Http Www Tandfonline Com Doi Pdf 10 1080
Http Www Tandfonline Com Doi Pdf 10 1080

The Surface Tension Of An Sds Solution As A Function Of Sds Download Scientific Diagram

Pdf Investigation On Some Properties Of Sds Solutions Semantic Scholar

Study Of Surface Properties Of Aqueous Solutions Of Sodium Dodecyl Sulfate In The Presence Of Hydrochloric Acid And Heavy Metal Ions Sciencedirect
Http Ijogst Put Ac Ir Article 9bae958e5b34c34cb026eada1 Pdf
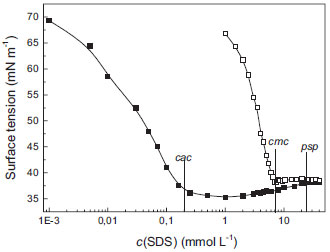
Quimica Nova Artigos
Http Ojs Wiserpub Com Index Php Fce Article Download Fce 9 14 52

Figure 1s Surface Tension Vs Log C Plots Of A Dpc B 9 1 Dpc Sds

Surface Tension And Adsorption Studies By Drop Profile Analysis Tensiometry Kairaliyeva 17 Journal Of Surfactants And Detergents Wiley Online Library

Surface Tension And Adsorption Studies By Drop Profile Analysis Tensiometry Kairaliyeva 17 Journal Of Surfactants And Detergents Wiley Online Library

Effect Of Impurities Upon The Surface Tension Of Sds Solutions Download Scientific Diagram

Surface Tension Of Pure Sds Solutions Circles And 0 01 Mixture Of Download Scientific Diagram

Figure 1 From Adsorption Layer Characteristics Of Mixed Sodium Dodecyl Sulfate C N Eo M Solutions 1 Dynamic And Equilibrium Surface Tension Semantic Scholar




